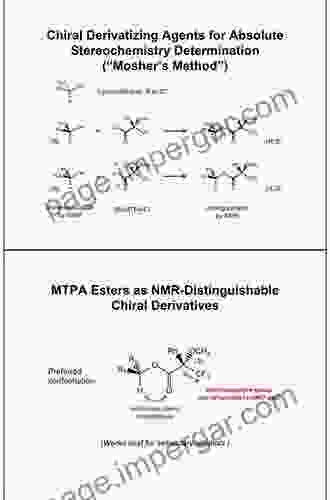
The Assignment of Absolute Configuration by NMR Using Chiral Derivatizing

In the intricate world of organic chemistry, determining the absolute configuration of chiral molecules is paramount to understanding their structure, reactivity, and biological function. Nuclear magnetic resonance (NMR) spectroscopy, a powerful analytical tool, plays a crucial role in this endeavor. By employing chiral derivatizing agents, chemists can harness the power of NMR to unveil the absolute configuration of chiral compounds.
This comprehensive guide delves into the principles and applications of NMR using chiral derivatizing agents. We will explore the theoretical foundations, practical considerations, and real-world examples to empower chemists with the knowledge and skills to confidently assign absolute configuration. Whether you are a seasoned researcher or a budding organic chemist, this definitive resource will illuminate the intricacies of this essential technique.
4.5 out of 5
| Language | : | English |
| File size | : | 37524 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Print length | : | 264 pages |
| Lending | : | Enabled |
Theoretical Foundations: NMR and Chiral Derivatizing
NMR spectroscopy relies on the magnetic properties of atomic nuclei to provide detailed information about the structure and dynamics of molecules. When placed in a magnetic field, certain nuclei, such as 1H and 13C, align with or against the field, resulting in different resonance frequencies. These frequencies correspond to the chemical environment of each nucleus, enabling the identification and characterization of different atoms and groups within a molecule.
Chiral molecules, which lack mirror symmetry, exist as two distinct enantiomers that are non-superimposable mirror images of each other. The absolute configuration of a chiral molecule refers to the spatial arrangement of its atoms or groups around the chiral center. Assigning the absolute configuration is crucial for understanding the molecule's properties and interactions.
Chiral derivatizing agents are molecules that contain a chiral center and can form diastereomeric derivatives with chiral analytes. By derivatizing the analyte with a chiral derivatizing agent, the two enantiomers of the analyte will react to form two distinct diastereomers. These diastereomers have different chemical environments, resulting in distinct NMR signals that can be used to assign the absolute configuration of the analyte.
Practical Considerations: Choosing the Right Chiral Derivatizing Agent
The choice of chiral derivatizing agent is critical for successful NMR analysis. Several factors need to be considered, including:
- Reactivity: The derivatizing agent should react efficiently with the analyte under mild conditions.
- Selectivity: The derivatizing agent should selectively derivatize the desired functional group(s) without interfering with other parts of the molecule.
- Stability: The derivatized product should be stable under the conditions of the NMR experiment.
- NMR properties: The derivatizing agent should introduce unique NMR signals that can be easily distinguished from the signals of the analyte.
Commonly used chiral derivatizing agents include:
- Mosher's acid and ester
- MTPA (α-methoxy-α-trifluoromethylphenylacetic acid)
- Evans' auxiliary
- CBS (Corey-Bakshi-Shibata) reagent
- BINAP (2,2'-bis(diphenylphosphino)-1,1'-binaphthyl)
Real-World Applications: Case Studies and Examples
The power of NMR using chiral derivatizing agents has been demonstrated in various fields of chemistry, including natural product isolation, pharmaceutical development, and stereoselective synthesis. Here are a few notable examples:
- Natural product isolation: In the isolation of the natural product taxol, NMR analysis using chiral derivatizing agents played a crucial role in determining the absolute configuration of several chiral centers within the molecule.
- Pharmaceutical development: In the development of the HIV protease inhibitor saquinavir, NMR using chiral derivatizing agents was used to assign the absolute configuration of the chiral intermediates, ensuring the synthesis of the correct enantiomer with the desired biological activity.
- Stereoselective synthesis: In the stereoselective synthesis of chiral compounds, NMR using chiral derivatizing agents can be used to monitor the progress of the reaction and determine the enantiomeric purity of the product.
The assignment of absolute configuration by NMR using chiral derivatizing agents is a powerful technique that has revolutionized the field of organic chemistry. By harnessing the principles of NMR spectroscopy and the versatility of chiral derivatizing agents, chemists can confidently determine the spatial arrangement of atoms and groups in chiral molecules. This knowledge is essential for understanding molecular structure, reactivity, and biological function. Whether you are a seasoned researcher or a budding organic chemist, this comprehensive guide provides the foundation and practical insights to master this essential technique.
4.5 out of 5
| Language | : | English |
| File size | : | 37524 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Print length | : | 264 pages |
| Lending | : | Enabled |
Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
 Book
Book Novel
Novel Page
Page Chapter
Chapter Text
Text Story
Story Genre
Genre Reader
Reader Library
Library Paperback
Paperback E-book
E-book Magazine
Magazine Newspaper
Newspaper Paragraph
Paragraph Sentence
Sentence Bookmark
Bookmark Shelf
Shelf Glossary
Glossary Bibliography
Bibliography Foreword
Foreword Preface
Preface Synopsis
Synopsis Annotation
Annotation Footnote
Footnote Manuscript
Manuscript Scroll
Scroll Codex
Codex Tome
Tome Bestseller
Bestseller Classics
Classics Library card
Library card Narrative
Narrative Biography
Biography Autobiography
Autobiography Memoir
Memoir Reference
Reference Encyclopedia
Encyclopedia Pierre Berton
Pierre Berton Tommy Dades
Tommy Dades Michael Mcgeary
Michael Mcgeary Prash Ganendran
Prash Ganendran Rita Y Shuler
Rita Y Shuler Rado Bohinc
Rado Bohinc Rhys A Martin
Rhys A Martin Richard A Layfield
Richard A Layfield Shuo Ma
Shuo Ma Rebecca Gregoire Lindenbach
Rebecca Gregoire Lindenbach Phoenix Kennedy
Phoenix Kennedy Peter A Ciullo
Peter A Ciullo Randy G Floyd
Randy G Floyd Ron Peterson Jr
Ron Peterson Jr R A Burt
R A Burt Stephen F Arno
Stephen F Arno Ryan Holiday
Ryan Holiday Pippa Mattinson
Pippa Mattinson Thomas J Salerno
Thomas J Salerno Richard Woodman
Richard Woodman
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Patrick HayesHow Two Million British Women Survived Without Men After the First World War
Patrick HayesHow Two Million British Women Survived Without Men After the First World War Jamie BlairFollow ·10.1k
Jamie BlairFollow ·10.1k Harold BlairFollow ·4.1k
Harold BlairFollow ·4.1k Logan CoxFollow ·4.5k
Logan CoxFollow ·4.5k Cody BlairFollow ·2.4k
Cody BlairFollow ·2.4k Jeff FosterFollow ·2.5k
Jeff FosterFollow ·2.5k Jan MitchellFollow ·6.7k
Jan MitchellFollow ·6.7k Chase SimmonsFollow ·4.7k
Chase SimmonsFollow ·4.7k Anthony BurgessFollow ·2.5k
Anthony BurgessFollow ·2.5k

 Branson Carter
Branson Carter"Flesh Wounds" by Richard Glover: A Provocative...
In his thought-provoking...

 Casey Bell
Casey BellTrial Techniques and Trials: Essential Knowledge for...
Navigating...

 Samuel Taylor Coleridge
Samuel Taylor ColeridgeUnravel the Mystery: Delve into the Expanded Annotated...
Immerse yourself in the captivating world...

 Amir Simmons
Amir SimmonsTrial Evidence Aspen Coursebook Series: Your Ultimate...
In the realm of litigation, evidence...

 Xavier Bell
Xavier BellThe Pursuit of Accountability: Achieving Success Through...
Are you tired of...
4.5 out of 5
| Language | : | English |
| File size | : | 37524 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Print length | : | 264 pages |
| Lending | : | Enabled |